ABOUT PLASMINOGEN
DEFICIENCY (PLGD-1)
This autosomal recessive disorder occurs when a person has a mutation in both copies of the gene for plasminogen.2
Lesions can form spontaneously but are typically triggered by local infection, irritation, injury, or surgical intervention.3
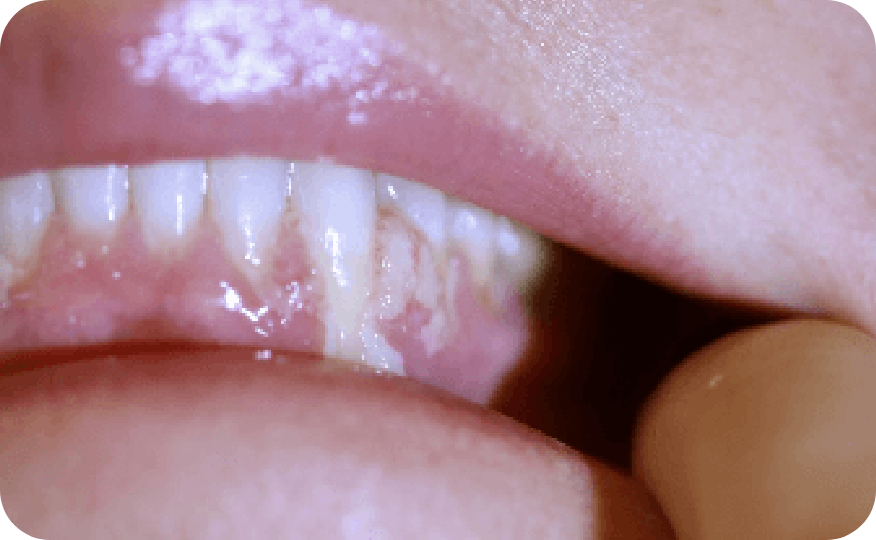
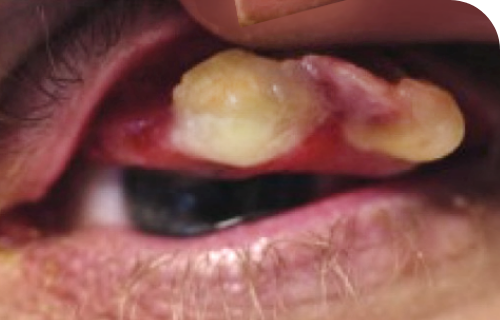
Ligneous gingivitis in a real patient with PLGD-14
Unresolved lesions can severely impact organ function and overall quality of life5
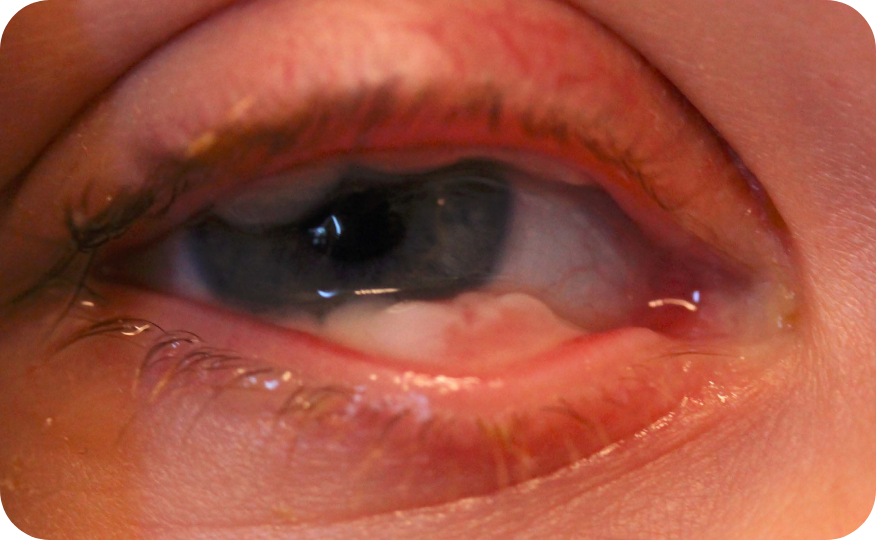
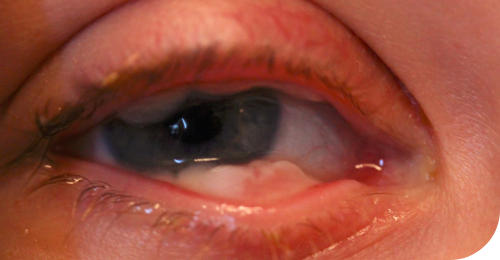
Ligneous conjunctivitis in a real patient with PLGD-14
Ligneous lesions often affect multiple organs with symptoms resembling more common conditions like chronic conjunctivitis, recurrent ear infections, or recurrent pneumonia. This, along with the rarity of the disease and variability in the initial point of care, contributes to the frequent misdiagnosis or underdiagnosis of PLGD-1.5
Consider PLGD-1 when common conditions don't respond to typical treatments.
Diagnosing PLGD-1
8 of 10 people with PLGD-1 have lesions in multiple organs6*
Eyes
Eyes
The earliest signs of ligneous conjunctivitis typically resemble other common ocular conditions like pink eye or pyogenic granulomas, but if left untreated can lead to vision loss or blindness.5
Hear a real patient storyMouth
Mouth
Ligneous gingivitis is typically painless but can lead to periodontal destruction (tissue loss) and ultimately tooth loss if left untreated.3
Hear a real patient storyEars
Ears
In the ear, lesions on the tympanic membrane or in the middle ear can cause impaired hearing or recurrent ear infections. Left untreated, lesions can lead to chronic ear infections, hearing loss, or other ear-related complications.3,5,7
Hear a real patient storyBrain
Brain
Lesions impacting the central nervous system can manifest as obstructive hydrocephalus due to fibrin deposition in the cerebral ventricular system causing a buildup of fluid. Dandy-Walker syndrome can also occur in people with PLGD-1.3
Respiratory Tract
Respiratory Tract
Lesions in the respiratory tract may initially present as recurrent pneumonia, asthma, or upper respiratory tract infections. If left untreated, lesions can cause airway obstruction, collapsed lung, or respiratory failure.5
Renal System
Renal System
Lesions in the renal tract may lead to kidney stones, blood in the urine, or impaired kidney function. Left untreated, this could progress to chronic kidney disease or kidney failure.5
Learn how to diagnose PLGD-1Female Reproductive Tract
Female Reproductive Tract
Lesions in the female genital tract can lead to extremely painful menstruation (dysmenorrhea), abnormal menses, amenorrhea, painful intercourse (dyspareunia), and infertility.3
Hear a real patient storyGastrointestinal Tract
Gastrointestinal Tract
Lesions in the GI tract can mimic inflammatory bowel disease, causing abdominal pain, ulcers, GI bleeding, or obstruction. Lesions can also develop in the esophagus, stomach, intestines, or rectum causing discomfort, difficulty swallowing, or changes in bowel habits.5,8
Learn how to test for PLGD-1Skin
Skin
When PLGD-1 impacts the skin, it often involves chronic lesions, ulcers, and delayed healing. It can also lead to a condition known as juvenile colloid milium, which is characterized by growths on areas of the skin exposed to the sun.3,9
Prevalence data continue to evolve and may change as additional PLGD-1 cases are identified and more research becomes available.
*Patient pool of 23 individuals that primarily originate from Turkey and various Arabian countries (61%). When compared to previously published cases, this group of patients presented at a younger mean age (1 year and 10 months old) and showed a high rate of consanguinity (61%).6
†Patient pool of 102 individuals with a mean age at onset of 5 years and 5 months and a consanguinity rate of 40%.6
‡Patient pool of 74 individuals with a median age at onset of 9.5 months.1
Ligneous conjunctivitis is the most common manifestation6
87% of people with PLGD-1 present with ligneous conjunctivitis6†
Ligneous conjunctivitis is a rare, chronic, and recurrent form of pseudomembranous conjunctivitis characterized by the formation of fibrinous pseudomembranes on the palpebral conjunctivae.10
Signs of LC include mucoid discharge, chronic tearing, and redness of the conjunctivae, often resembling other ocular conditions like bacterial conjunctivitis, pyogenic granuloma, conjunctival papilloma, or inclusion cysts.5,10
Symptoms may progress to the formation of pseudomembranes on the palpebral conjunctivae, eventually leading to mucosal thickening with a wood-like (ligneous) consistency that replaces the normal eyelid mucosa.1
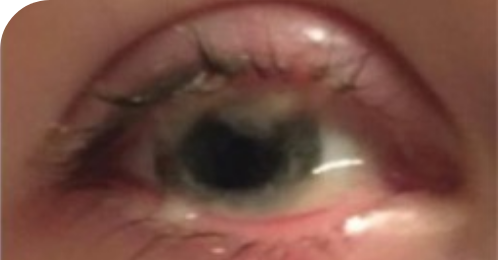
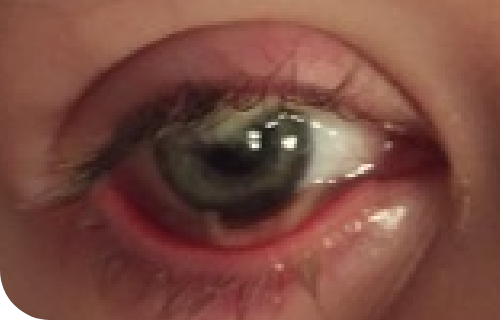
Insights into PLGD-1, LC, and RYPLAZIM
Learn more about the systemic nature of PLGD-1, the varied presentations of LC, and RYPLAZIM efficacy through patient case studies and the LC Explorer
Explore here
Ligneous lesions vary widely in appearance
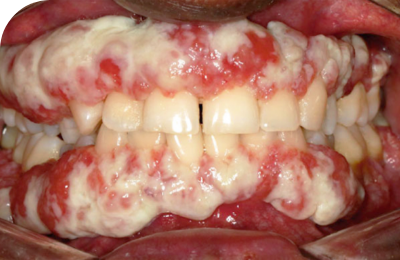
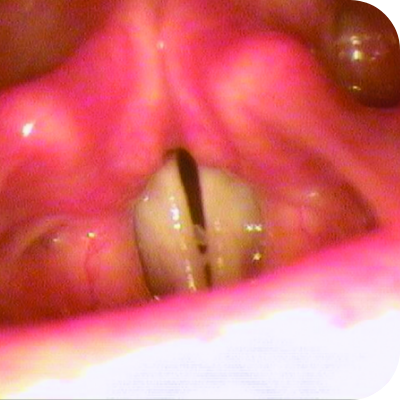
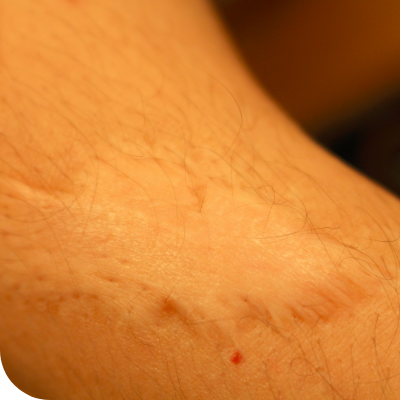
Lesions due to PLGD-1 may present in a variety of ways, from watery, stringy pseudomembranes to thick wood-like lesions.3
Not only does symptom severity fluctuate over time, but the overall severity of the disorder varies greatly from patient to patient depending on the location and duration of lesions. Internal lesions can even occur without clinical suspicion.3,5
Even members of the same family impacted by PLGD-1 can present with drastically different manifestations.3
Review the lesion image gallery
Unresolved lesions can have severe impacts9:
Blindness
Life-threatening airway complications
Tooth Loss
Periodontal destruction
Compromised organ function
Organ system failure
Infertility
Hearing loss
Reduced quality of life
INDICATIONS AND USAGE
RYPLAZIM® (plasminogen, human-tvmh) is a plasma-derived human plasminogen indicated for the treatment of patients with plasminogen deficiency type 1 (hypoplasminogenemia).
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS:
RYPLAZIM is contraindicated in patients with known hypersensitivity to plasminogen or other components of RYPLAZIM.
WARNINGS AND PRECAUTIONS:
- Bleeding: RYPLAZIM administration may lead to bleeding at active mucosal disease-related lesion sites or worsen active bleeding not related to disease lesions. Discontinue RYPLAZIM if serious bleeding occurs. Monitor patients during and for 4 hours after infusion when administering RYPLAZIM to patients with bleeding diatheses and patients taking anticoagulants, antiplatelet drugs, or other agents which may interfere with normal coagulation.
- Tissue Sloughing: Respiratory distress due to tissue sloughing may occur in patients with mucosal lesions in the tracheobronchial tree following RYPLAZIM administration. Please monitor appropriately.
- Transmission of Infectious Agents: RYPLAZIM is made from human plasma and therefore carries a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and theoretically, the Creutzfeldt-Jakob Disease (CJD) agent.
- Hypersensitivity Reactions: Hypersensitivity reactions, including anaphylaxis, may occur with RYPLAZIM. If symptoms occur, discontinue RYPLAZIM and administer appropriate treatment.
- Neutralizing Antibodies: Neutralizing antibodies (inhibitors) may develop, although they were not observed in clinical trials. If clinical efficacy is not maintained (e.g., development of new or recurrent lesions), determine plasminogen activity trough levels in plasma.
- Laboratory Abnormalities: Patients receiving RYPLAZIM may have elevated blood levels of D-dimer. D-dimer levels will lack interpretability in patients being screened for venous thromboembolism (VTE).
ADVERSE REACTIONS:
The most frequent (incidence ≥ 10%) adverse reactions in clinical trials were abdominal pain, bloating, nausea, fatigue, extremity pain, hemorrhage, constipation, dry mouth, headache, dizziness, arthralgia, and back pain.
To report SUSPECTED ADVERSE REACTIONS, contact KEDRION at 1-855-427-6378 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Click here for the RYPLAZIM Full Prescribing Information.
This site is intended for residents of the US only.
References:
1. Schuster V, Hügle B, Tefs K. Plasminogen deficiency. J Thromb Haemost. 2007;5(12):2315-2322.
2. Tefs K, Gueorguieva M, Klammt J, et al. Molecular and clinical spectrum of type I plasminogen deficiency: a series of 50 patients. Blood. 2006;108(9):3021-3026.
3. Congenital type 1 plasminogen deficiency. NORD. Updated Februrary 6, 2025. Accessed July 16, 2025. https://rarediseases.org/rare-diseases/congenital-plasminogen-deficiency
4. Data on file. Kedrion Biopharma Inc.
5. Shapiro AD, Nakar C. How I treat type 1 plasminogen deficiency. Blood. 2025;145(25):2954-2965.
6. Klammt J, Kobelt L, Aktas D, et al. Identification of three novel plasminogen (PLG) gene mutations in a series of 23 patients with low PLG activity. Thromb Haemost. 2011;105(3):454-460.
7. Mehta R, Shapiro AD. Plasminogen deficiency. Haemophilia. 2008;14(6):1261-1268.
8. Balram B, Thiesen A, Kroeker KI. Inflammatory bowel disease: a gastrointestinal presentation of congenital plasminogen deficiency. ACG Case Rep J. 2021;8(5):e00613.
9. Shapiro AD, Menegatti M, Palla R, et al. An international registry of patients with plasminogen deficiency (HISTORY). Haematologica. 2020;105(3):554-561.
10. Schuster V, Seregard S. Ligneous conjunctivitis. Surv Ophthalmol. 2003;48(4):369-388.
11. Sadasivan A, Ramesh R, Mathew DG. Ligneous periodontitis in a patient with type 1 plasminogen deficiency: a case report and review of the literature. Case Rep Dent. 2020;2020:5680535.

