ABOUT RYPLAZIM
How does RYPLAZIM work?
RYPLAZIM temporarily restores plasminogen levels which re-establishes fibrinolytic activity, allowing the body to break down fibrin-rich lesions on mucous membranes throughout the body.1,2
Proven Efficacy
RYPLAZIM demonstrated clinical success at 48 weeks1,3*

ALL patients achieved overall clinical success*
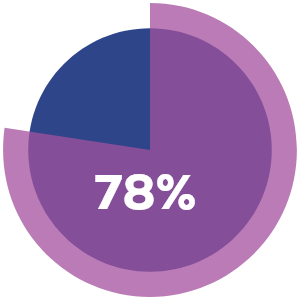 External
Externallesions
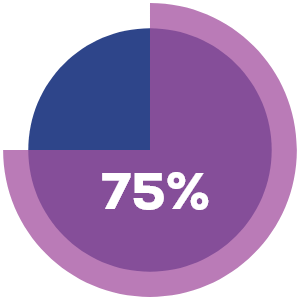 Internal
Internallesions
% of lesions resolved completely after week 48
Median time to complete lesion resolution was only 8 weeks

new or recurrent lesions in any patient through week 48
*Clinical success defined as 50% of patients with visible or other measurable non-visible lesions achieving at least 50% improvement in lesion number/size or functionality impact from baseline.
Ligneous conjunctivitis BEFORE and DURING RYPLAZIM4
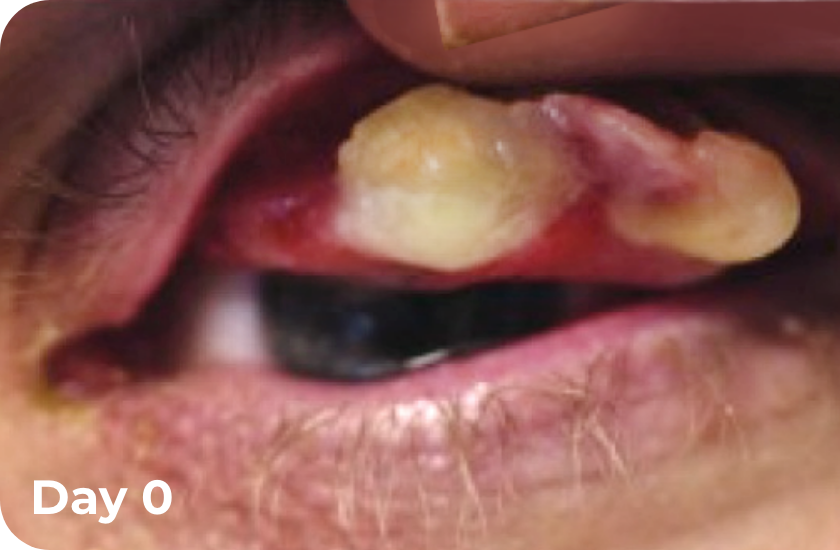
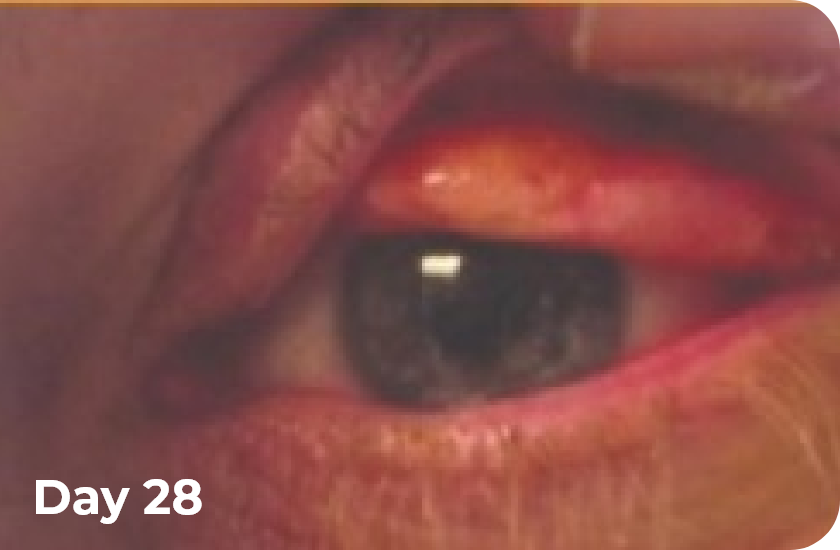
*Includes data from 2 clinical studies and the RYPLAZIM expanded access and compassionate use programs.
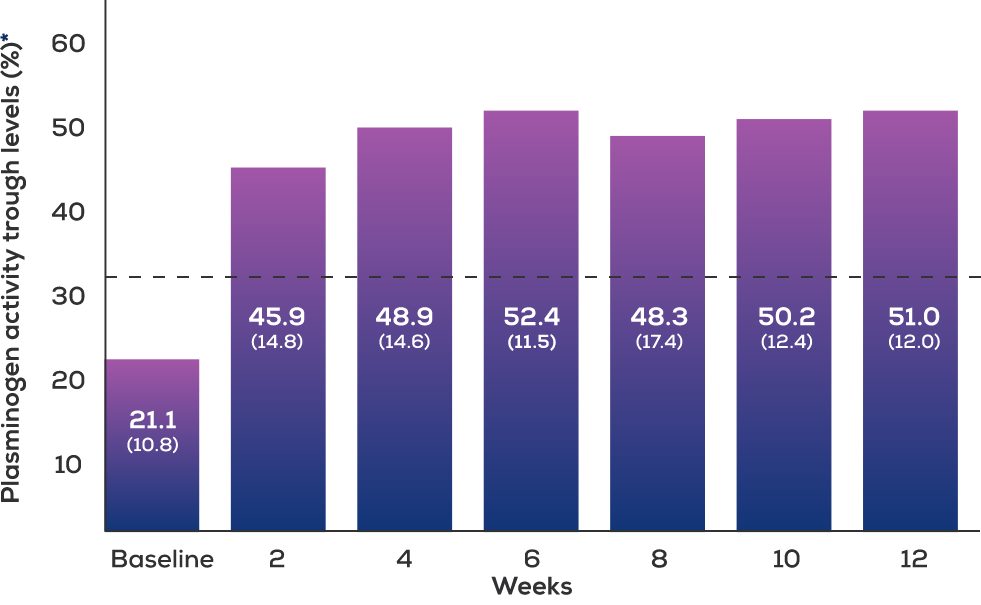
After the first infusion, plasminogen activity levels reached physiological levels,* were sustained for 24 hours, and remained an absolute 10% above baseline at 72 hours.
After 12 weeks, plasminogen activity levels remained an absolute 10% above baseline at 96 hours.
*Physiological mean and absolute plasminogen activity: 70%-130%
Over ~5 years, a separate study validated safety and efficacy of RYPLAZIM as maintenance therapy5
12 patients with PLGD-1 were observed, and the key clinical findings included5:
Treatment with RYPLAZIM was well tolerated
No treatment-emergent adverse events (TEAEs) required the permanent discontinuation of RYPLAZIM
New or recurrent lesions observed in any patient while adhering to their prescribed treatment regimen†
Plasminogen activity levels remained adequate under proper dosing protocols
Anti-plasminogen antibody development was not observed
Lesion resolution relies on consistent dosing5
4/12 patients experienced lesion recurrence
following reduced or missed doses.
ALL patients improved
after resuming recommended RYPLAZIM dosing, resulting in lesion control in all 12 patients.
†This study included use of RYPLAZIM at different dosing intervals than what appears in the RYPLAZIM Prescribing Information.
RYPLAZIM is the result of an innovative manufacturing process that promotes viral clearance1
Plasma collection1,6
Every plasma donor is carefully screened at FDA-licensed plasma donation centers.
Only qualified donors provide plasma for RYPLAZIM manufacturing.
Affinity
Chromatography
Solvent/detergent
treatment
20 nm
nanofiltration
INDICATIONS AND USAGE
RYPLAZIM® (plasminogen, human-tvmh) is a plasma-derived human plasminogen indicated for the treatment of patients with plasminogen deficiency type 1 (hypoplasminogenemia).
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS:
RYPLAZIM is contraindicated in patients with known hypersensitivity to plasminogen or other components of RYPLAZIM.
WARNINGS AND PRECAUTIONS:
- Bleeding: RYPLAZIM administration may lead to bleeding at active mucosal disease-related lesion sites or worsen active bleeding not related to disease lesions. Discontinue RYPLAZIM if serious bleeding occurs. Monitor patients during and for 4 hours after infusion when administering RYPLAZIM to patients with bleeding diatheses and patients taking anticoagulants, antiplatelet drugs, or other agents which may interfere with normal coagulation.
- Tissue Sloughing: Respiratory distress due to tissue sloughing may occur in patients with mucosal lesions in the tracheobronchial tree following RYPLAZIM administration. Please monitor appropriately.
- Transmission of Infectious Agents: RYPLAZIM is made from human plasma and therefore carries a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and theoretically, the Creutzfeldt-Jakob Disease (CJD) agent.
- Hypersensitivity Reactions: Hypersensitivity reactions, including anaphylaxis, may occur with RYPLAZIM. If symptoms occur, discontinue RYPLAZIM and administer appropriate treatment.
- Neutralizing Antibodies: Neutralizing antibodies (inhibitors) may develop, although they were not observed in clinical trials. If clinical efficacy is not maintained (e.g., development of new or recurrent lesions), determine plasminogen activity trough levels in plasma.
- Laboratory Abnormalities: Patients receiving RYPLAZIM may have elevated blood levels of D-dimer. D-dimer levels will lack interpretability in patients being screened for venous thromboembolism (VTE).
ADVERSE REACTIONS:
The most frequent (incidence ≥ 10%) adverse reactions in clinical trials were abdominal pain, bloating, nausea, fatigue, extremity pain, hemorrhage, constipation, dry mouth, headache, dizziness, arthralgia, and back pain.
To report SUSPECTED ADVERSE REACTIONS, contact KEDRION at 1-855-427-6378 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Click here for the RYPLAZIM Full Prescribing Information.
This site is intended for residents of the US only.
References:
1. RYPLAZIM [prescribing information]. Kedrion Biopharma Inc. 2024.
2. Schuster V, Hügle B, Tefs K. Plasminogen deficiency. J Thromb Haemost. 2007;5(12):2315-2322.
3. Shapiro AD, Nakar C, Parker JM, Thibaudeau K, Crea R, Sandset PM. Plasminogen, human-tvmh for the treatment of children and adults with plasminogen deficiency type 1. Haemophilia. 2023;29(6):1556-1564.
4. Data on file. Kedrion Biopharma Inc.
5. Shapiro AD, McDaniel H, Decker RW, et al. Safety and efficacy of long-term treatment of type 1 plasminogen deficient patients with intravenous plasminogen replacement therapy. Haemophilia. 2025;31(3):477-484.
6. International Quality Plasma Program (IQPP). Plasma Protein Therapeutics Association. Accessed December 10, 2025. https://www.pptaglobal.org/material/international-quality-plasma-program-iqpp