Dosing for sustained systemic lesion resolution for patients with PLGD-11,2
FDA-approved dose: 6.6 mg/kg every 2-4 days1
Proper dosing ensures that plasminogen is consistently replaced, allowing the body to break down fibrin and resolve lesions—as long as therapeutic levels are maintained.1,2
How does RYPLAZIM work?
RYPLAZIM temporarily replaces plasminogen in the blood1
Fibrinolytic activity is restored3
Fibrin-rich lesions resolve1
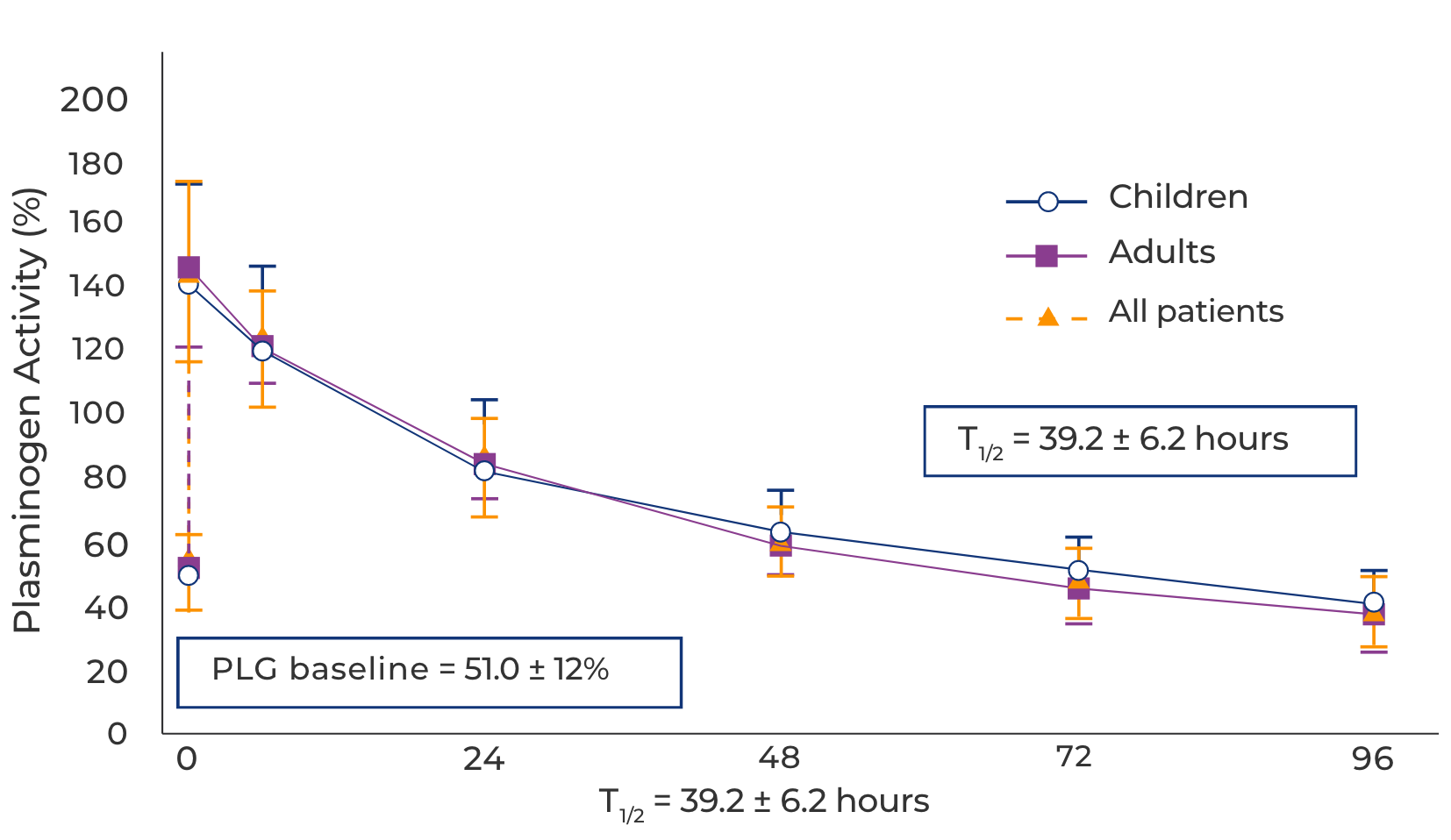
The pharmacokinetics of RYPLAZIM support dosing every 2-4 days1
Pharmacokinetic profile following week 12 RYPLAZIM infusion4
Inconsistent dosing can lead to lesion recurrence2
In the long-term 5-year study, 4/12 patients experienced lesion recurrence following reduced or missed doses.
ALL patients improved after resuming recommended RYPLAZIM dosing, resulting in lesion control in all 12 patients.
Number of vials open 0 Volume to infuse 0 mL
Dose calculation formulas:
1. Infusion volume (mL) = body weight (kg) x 1.2
2. Number of vials = Infusion volume (mL) x 0.08
frequency of infusions -
See the chart in the 72 hours tab for dosing frequency guidance and calculator formulas.
Initiating treatment with RYPLAZIM
Administer via intravenous (IV) infusion in a clinical setting or at home with the proper training and guidance1
Before the first dose of RYPLAZIM1
Obtain baseline plasminogen activity level (if patient is receiving fresh frozen plasma,
allow for a 7-day washout period before obtaining baseline level)
The first dose1
1. Initiate dosing at a frequency of every 3 days
2. If your patient has suspected or confirmed lesions in the respiratory tract, it is recommended to initiate treatment with RYPLAZIM in a clinical setting to closely monitor for signs of respiratory distress due to tissue sloughing. Tissue sloughing is an expected response to RYPLAZIM, but can result in bleeding or organ obstruction.
72 hours after dose 1 and before dose 21
1. Obtain a trough plasminogen activity level
2. Adjust dose frequency according to the chart below
3. Maintain this dosing frequency for 12 weeks while treating active lesions
Trough plasminogen activity levels
(Absolute increase above
baseline at 72 hours)
Recommended dosing frequency
<10%*
Every 2 days
≥10% but ≤20%*
Remains every 3 days
≥20%*
Every 4 days
*Absolute change in plasminogen activity (%)
Use the frequency calculator to determine the right dosing interval for your patient using their trough plasminogen activity levels.
Frequency calculatorIf desired clinical response occurs by week 121
Continue at the same dosing frequency and monitor for new or recurrent lesions every 12 weeks.
If desired clinical response does not occur by week 121
1. Increase dosing frequency in 1-day increments every 4-6 weeks (up to every other day) until lesions resolve or stabilize
2. Check trough plasminogen activity level:
- If ≥10%* above baseline, consider other treatment options, such as surgical removal of lesion in addition to plasminogen treatment
- If <10%,* confirm with a second trough activity level. If confirmed in combination with no clinical improvement, consider discontinuing plasminogen treatment due to the possibility of neutralizing antibodies†
*Absolute change in plasminogen activity (%)
†Neutralizing antibodies (inhibitors) were not observed during the clinical trials but it is possible they may develop
Dose adjustments may be needed for the following circumstances1,5:
Weight change
Lesion recurrence
New symptoms
Unavoidable surgical intervention
Lifestyle changes
Pregnancy*
*Extra activity level check
RYPLAZIM can be administered1
By the patient
if they have been taught how to infuse RYPLAZIM
By a caregiver
at home
By a nurse
at a healthcare facility, like a hospital or a clinic
Managing long-term treatment with RYPLAZIM
Have a patient with PLGD-1? You don't have to manage it alone.
Due to the systemic nature of the disease, caring for people with PLGD-1 will likely require coordinated care from healthcare providers across multiple disciplines, with a hematologist serving as the central point of care5
Coordinate with a hematologist
Request an individualized pharmacokinetic (PK) study for your patient
Prepare a PK-tailored treatment plan
Activate the app for your patient
With the app, patients can:
record and track their infusions
see their predicted activity level in real time
see their estimated future levels
receive reminders when it’s time for an infusion
receive notifications whenever levels drop to the low zone
INDICATIONS AND USAGE
RYPLAZIM® (plasminogen, human-tvmh) is a plasma-derived human plasminogen indicated for the treatment of patients with plasminogen deficiency type 1 (hypoplasminogenemia).
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS:
RYPLAZIM is contraindicated in patients with known hypersensitivity to plasminogen or other components of RYPLAZIM.
WARNINGS AND PRECAUTIONS:
- Bleeding: RYPLAZIM administration may lead to bleeding at active mucosal disease-related lesion sites or worsen active bleeding not related to disease lesions. Discontinue RYPLAZIM if serious bleeding occurs. Monitor patients during and for 4 hours after infusion when administering RYPLAZIM to patients with bleeding diatheses and patients taking anticoagulants, antiplatelet drugs, or other agents which may interfere with normal coagulation.
- Tissue Sloughing: Respiratory distress due to tissue sloughing may occur in patients with mucosal lesions in the tracheobronchial tree following RYPLAZIM administration. Please monitor appropriately.
- Transmission of Infectious Agents: RYPLAZIM is made from human plasma and therefore carries a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and theoretically, the Creutzfeldt-Jakob Disease (CJD) agent.
- Hypersensitivity Reactions: Hypersensitivity reactions, including anaphylaxis, may occur with RYPLAZIM. If symptoms occur, discontinue RYPLAZIM and administer appropriate treatment.
- Neutralizing Antibodies: Neutralizing antibodies (inhibitors) may develop, although they were not observed in clinical trials. If clinical efficacy is not maintained (e.g., development of new or recurrent lesions), determine plasminogen activity trough levels in plasma.
- Laboratory Abnormalities: Patients receiving RYPLAZIM may have elevated blood levels of D-dimer. D-dimer levels will lack interpretability in patients being screened for venous thromboembolism (VTE).
ADVERSE REACTIONS:
The most frequent (incidence ≥ 10%) adverse reactions in clinical trials were abdominal pain, bloating, nausea, fatigue, extremity pain, hemorrhage, constipation, dry mouth, headache, dizziness, arthralgia, and back pain.
To report SUSPECTED ADVERSE REACTIONS, contact KEDRION at 1-855-427-6378 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Click here for the RYPLAZIM Full Prescribing Information.
This site is intended for residents of the US only.
References:
1. RYPLAZIM [prescribing information]. Kedrion Biopharma Inc. 2024. 2. Shapiro AD, McDaniel H, Decker RW, et al. Safety and efficacy of long-term treatment of type 1 plasminogen deficient patients with intravenous plasminogen replacement therapy. Haemophilia. 2025;31(3):477-484. 3. Schuster V, Hügle B, Tefs K. Plasminogen deficiency. J Thromb Haemost. 2007;5(12):2315-2322. 4. Shapiro AD, Nakar C, Parker JM, et al. Plasminogen replacement therapy for the treatment of children and adults with congenital plasminogen deficiency. Blood. 2018;131(12):1301-1310. 5. Shapiro AD, Nakar C. How I treat type 1 plasminogen deficiency. Blood. 25;145(25):2954-2965. 6. WAPPS-Hemo homepage. Accessed December 11, 2025. https://www.wapps-hemo.org/Default.aspx 7. What is mywapps? myWAPPS App. Accessed December 11, 2025. https://mywapps.org



